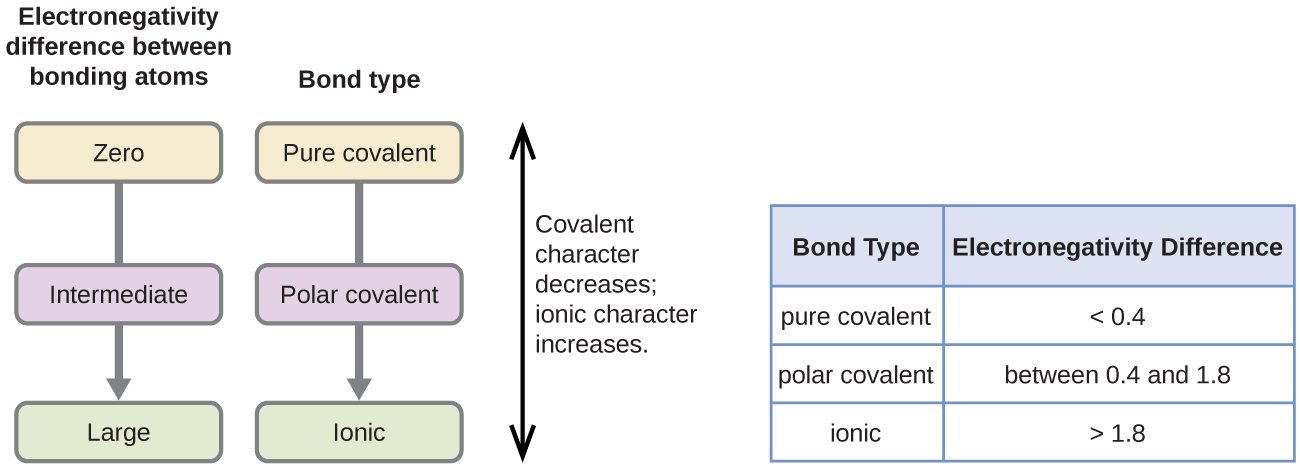
Ionic Polar Covalent Nonpolar Covalent Chart
The greater the difference in electronegativity between two atoms the more polar their bond is. Atoms with similar EN.
7 2 Covalent Bonding Chemistry
Molecules whose atoms have equal or nearly equal electronegativities have zero or very small dipole moments.

. F F D en 0 nonpolar covalent H F D en 19 polar covalent LiF D en 30 ionic Ionic Bonds Polar Covalent Bonds Nonpolar Covalent Bonds. Use a conductivity sensor to determine if an unknown substance is an ionic polar covalent or non-polar covalent compound based on its physical properties. Non-polar molecules are of two types.
Sodium chloride NaCl is polar with ionic bond. Start studying Ionicpolar Covalent Non Polar Covalent Chem Chart. Bonds can be found with a range of polarities from completely ionic to completely covalent.
Arrow_forward Explain the differences among nonpolar covalent bonds polar covalent bonds and ionic bonds. The rule is that when the electronegativity level is greater than 2 the bond is considered ionic. Remind the class that covalent substances are either polar not symmetrical the molecule has two different ends.
Nonpolar Not all covalent compounds are categorized as polar. When the difference is very small or zero the bond is covalent and nonpolar. 5 Polar Covalent Bond This is a type of covalent bond.
The absolute values of the electronegativity differences between the atoms in the bonds HH HCl and NaCl are 0 nonpolar 09 polar covalent and 21 ionic respectively. Ionic Bonds are formed by ELECTROSTATIC ATTRACTION between positive and negative ions and POLAR COVALENT TRANSFERElectrons between atom bonds Inorganic compounds such as acids and salts have Ionic Bonds Covalent Bonds are NOT CONDUCTORS PROPERTIES Ionic Bonds have charged ions in gas liquid and solid Ionic Bonds in SOLIDS are NOT GOOD. The bond is ionic.
Properties of Ionic and Covalent Compounds. There absolute values of the electronegativity differences between the atoms in the bonds are shown below. Ionic or bartleby.
When the difference is very small or zero the bond is covalent and nonpolar. When it is large the bond is polar covalent or ionic. CH bonds relatively nonpolar C-O C-X bonds moreelectronegative elements are polarelectronegative elements are polar.
The higher the. Briefly cite the main differences between ionic covalent and metallic bonds. Nonpolar covalent bonds with equal sharing of the bond electrons arise when the electronegativities of the two atoms are equal.
Here are a number of highest rated Nonpolar Covalent pictures on internet. For example a carbon-hydrogen bond is made. When the level is less than 5 it is a non-polar covalent bond.
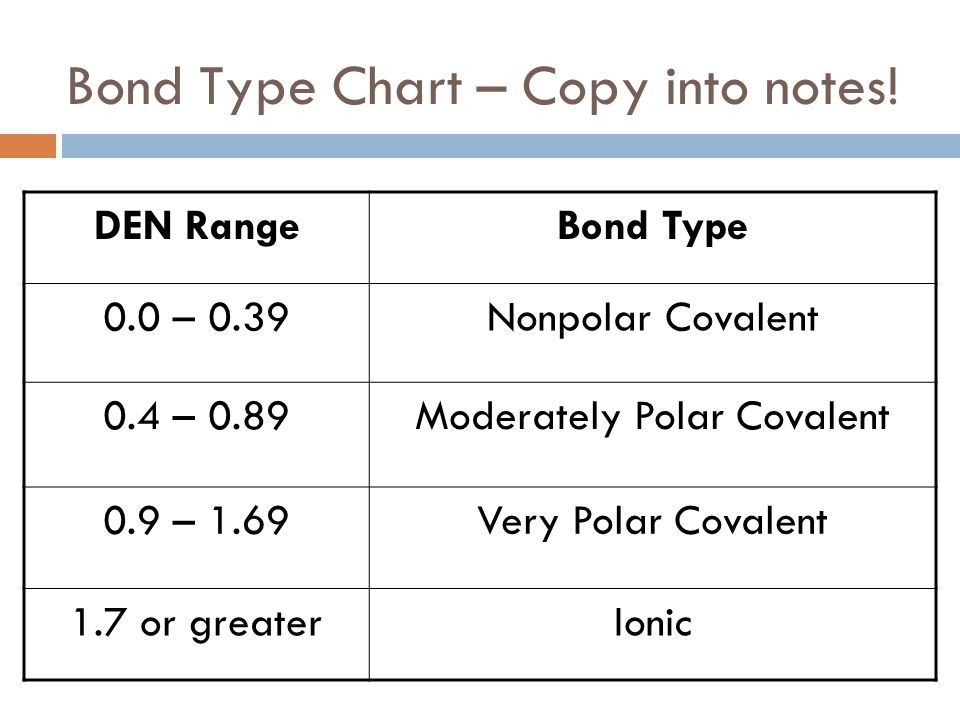
Definition of Electronegativity Electronegativity is a measure of how much an atom attracts an electron. The bond is nonpolar covalent If the difference is between 04-17 Some books say 19. Constituent polar and nonpolar covalent compounds.
Polar or Positive Atoms end. When will a bond be polar. Ionic or covalently bonded and to tape a paper label with the name and formula for each under the correct category sign on the board.
NaCl ΔEN 21 ionic bonding. HH ΔEN 0 nonpolar covalent or pure covalent bonding HCl ΔEN 09 polar covalent and. Polar covalent bond Formed between atoms having a difference in their electronegativities.
These molecules are symmetrical. Up to 24 cash back Polar vs. Finally if the bond is between 5 and 2 is a polar covalent bond.
Non-polar covalent bond Formed by identical atoms. Polar colavent bonding electrons shared unequally. Covalent bonds formed between atoms of the same kind are usually non-polar and the shared electrons are equidistant from both.
Is NaCl Polar Or Nonpolar Covalent Bond. Answers to chart on ionic-polar covalent and nonpolar covalentpdf. The bond is polar covalent If the difference is greater than 17 or above 20 in some books.
Bond Polarity and Inductive EffectBond Polarity and Inductive Effect. 5 Tests to Classify a Substance as Molecular Polar Non-Polar Ionic Metallic or Covalent Network is published by Ernest Wolfe in countdowneducation. Difference in EN 2.
Ionic November 11 2019 by Bozeman Science Leave a Comment Mr. We endure this nice of Nonpolar Covalent graphic could possibly be the most trending subject in the same way as we share it in google pro or facebook. Up to 24 cash back If the difference is between 00-03.
H 2 O HI HCl NH 3. When it is large the bond is polar covalent or ionic. Background Knowledge Solid Sodium iodide Nal Lactose C 0 Iodine I Tin Sn Bond Type Ionic Polar covalent Nonpolar covalent Metallic Melting Point oc 203 114 Color White White Dark gray Solubility in Water 25 Soluble Soluble Slightly soluble Insoluble 232 Shiny gray 1.
Water is an example of a molecule that has polar covalent bonds and engages in hydrogen bonding. Tonic Palar K Hydrophilic K and Cl Covalent Non Polar C and C O and P H Hydrachilic N and H Kovalent Polar Ksvalent Polar Covalent Non C and O H and C THydrophilic. We identified it from honorable source.
Its submitted by running in the best field. Ionic Polar Covalent Non-Polar Covalent. 7 rows The greater the electronegativity difference the more ionic the bond is.
Learn vocabulary terms and more with flashcards games and other study tools. Up to 24 cash back chart which will be used to distinguish and identify the unknown solids. To know in detail about the chemical formula of common compunds subscribe BYJUS.
Bonds that are partly ionic are called polar covalent bonds. Up to 24 cash back Polar and Nonpolar Covalent Bonds Chemical bonds exist along a continuum. Up to 24 cash back Polar Nonpolar and Ionic Bonding Information.
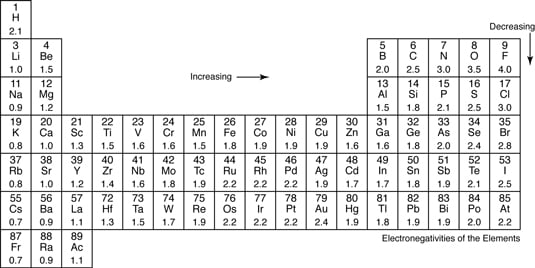
Polarity of covalent bonds Closertogether on the periodic table less polar bond Furtherapart on the periodic table more polar bond Molecules are called dipoles Ionic bonds are extremely polar beyond covalent. Difference in EN of atoms 2. In Biological systems polar covalent bonds are important because they allow the formation of another kind of weak bond called a hydrogen bond.
Andersen shows you how to determine if a bond is nonpolar covalent polar covalent or ionc. A second type of non-polar molecule has polar bonds but the molecular geometry is symmetrical allowing the bond dipoles to cancel. EgCl 2 O 2 N 2 etc.
Water sugar vinegar ammonia or nonpolar symmetrical both ends of the molecule are the same.
Chemystery Electronegativity And Polarity
Electronegativity And Polar Covalent Bonding Dummies
Solved This Question Is Already Answered The Signs In Blue Chegg Com
Comments
Post a Comment